The potential of nanomedicine to provide therapeutic benefits over conventional drug formulations has been proven with other drugs in the market. One example of success is DOXIL®, a liposome-based doxorubicin formulation which improves pharmacokinetic profile. In the same direction, DIVERSA aims to bridge the gap and to help other molecules reach later development stages. With our patented lipidic nanoemulsion, DIVERSA can offer an alternative solution to poorly soluble compounds and redefine the medicinal chemistry/lead screening workflow. DIVERSA can also help biomedical researchers reach preclinical stages by overcoming the barriers of solubility, permeability, and intracellular delivery of drug candidates.

HOW CAN DIVERSA HELP MED CHEM SCIENTISTS AND BIOMEDICAL RESEARCHERS?
One of the biggest challenges that arises between discovery stages and product development of small molecules is their low solubility. Most of the time, the nature of these new molecules, that will potentially be considered drug candidates, is highly lipophilic. Depending on the administration route, lipophilicity can be a problem when considering formulation development, and thus, clinical translation. In this sense, medicinal chemistry becomes key in trying to modulate the lipophilicity without compromising pharmacological activity: introducing polar functional groups or converting these molecules to salts are two strategies widely used.
FINDING THE BALANCE
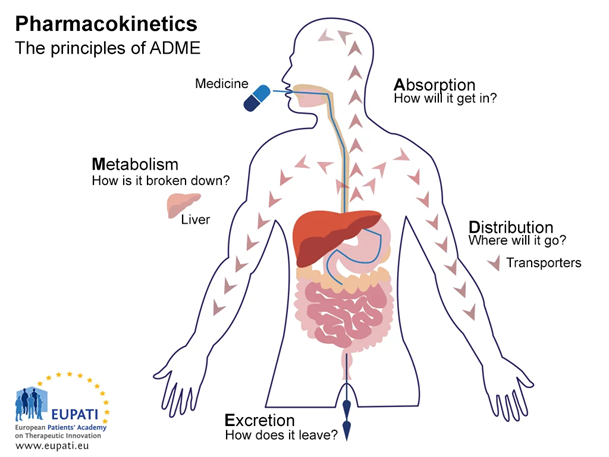
The equilibrium between lipophilicity and hydrophilicity needed to achieve a good pharmacological effect of a drug will dictate the ADME (Absorption, Distribution, Metabolism and Excretion) process and is, of course, dependent on the administration route.
PRECLINICAL FORMULATION
The other big branch that comes into play is the preclinical formulation work. By using different formulation strategies (i.e.: solutions, suspensions, amorphous solid dispersions…), it is possible to enhance the applicability of these early-stage compounds.
Encapsulation of small molecules into nanoparticles is another strategy used when wanting to modulate ADME properties. DIVERSA has developed a solution in this sense: a high-loading technology capable of encapsulating lipophilic small molecules based on the solubility of the drug in the two main excipients of the nanoemulsion.
Researchers can now benefit from this technology using the DIVTECH Small Molecule Kit, a ready-to-use kit to efficiently achieve the intracellular delivery of small molecules, or they can contact DIVERSA for a custom-made delivery solution for specific requirements through a co-development service. Furthermore, the association of a fluorophore to the nanoemulsions also allows for tracking.
FAVOURING DELIVERY
When considering systemic routes of administration (i.e., injection directly to the blood stream, such as intravenous – i.v. – Administration), the lipophilicity of a drug will dictate how the drug is distributed, metabolized, and excreted. In this sense, nanoparticles such as DIVTECH can provide solutions in terms of passive targeting to solid tumors (based on Enhanced Permeation and Retention – EPR – effect); as well as active targeting (i.e., by adding targeting moieties), improving therapeutic outcomes.
When considering other parenteral routes of administration, such as intraperitoneal (i.p.), intramuscular (i.m.), or subcutaneous (s.c.), DIVTECH can also enhance absorption processes by increasing bioavailability (i.e.: percentage of the administered dose that reaches the systemic circulation).
WOULD YOU LIKE TO TRY OUT OUR TECHNOLOGY?
We offer easy-to-use kits for small molecules association.
- DIVTECH Small Molecule Kit: improve the solubility and intracellular delivery of your small molecule candidate
- DIVTECH FluoGreen Small Molecule Kit: specifically designed to track drug-DIVTECH association
DIVERSA has also developed specific DIVETCH solutions for other types of therapeutic molecules such as proteins and peptides.
PREVIOUS BLOG ENTRIES:
Improving the fate of protein therapeutics