Lipid nanoemulsions
Our nanoparticle technology is the most versatile in the market
Reliable and efficient
Our nanoparticle emulsions are a patented product, with a stable and controlled production
We offer plenty of benefits to your molecule with the highest quality of emulsions. From hydrophobic to amphiphilic molecules, small or complex, we stabilise them and carry them to their target site.
Biocompatible
With different cell types and in vivo models like mice, rat and zebrafish
Stable
In different biological fluids, sera and cell culture media
High loading capacity
Ensuring the desired therapeutic effect
Efficient intracellular delivery
Overcoming biological barriers
Versatile
Able to carry a wide range of molecules
Applications
Nanoemulsion production
Assays
In vitro assays
- Labeling of live cells
- Tracking cell internalization
- FRET studies
- Intracellular delivery of therapeutic compounds
- Therapeutic evaluation
- Cytotoxicity studies
In vivo assays
- Biodistribution evaluation
- Therapeutic evaluation
- Cell tracking
Drug delivery of
Small molecules
Proteins
Peptides
Nucleic acids
Technical data
The facts and figures behind our nanoparticles
Our proprietary technology is based on lipidic emulsions with an easily controllable size and a narrow distribution. Our nanometric emulsions show great stability in different relevant biological fluids and biocompatibility with different cell types as well as in vivo models such as mouse, rat and zebrafish. They have high loading capacity and are versatile and capable of associating a wide variety of compounds as hydrophobic or amphiphilic small molecules, peptides, proteins, and oligonucleotides (such as DNA and RNA) showing great association efficacy and deliver them to their target site by overcoming biological barriers. DIVERSA’s technology can efficiently achieve the intracellular delivery of therapeutic molecules.
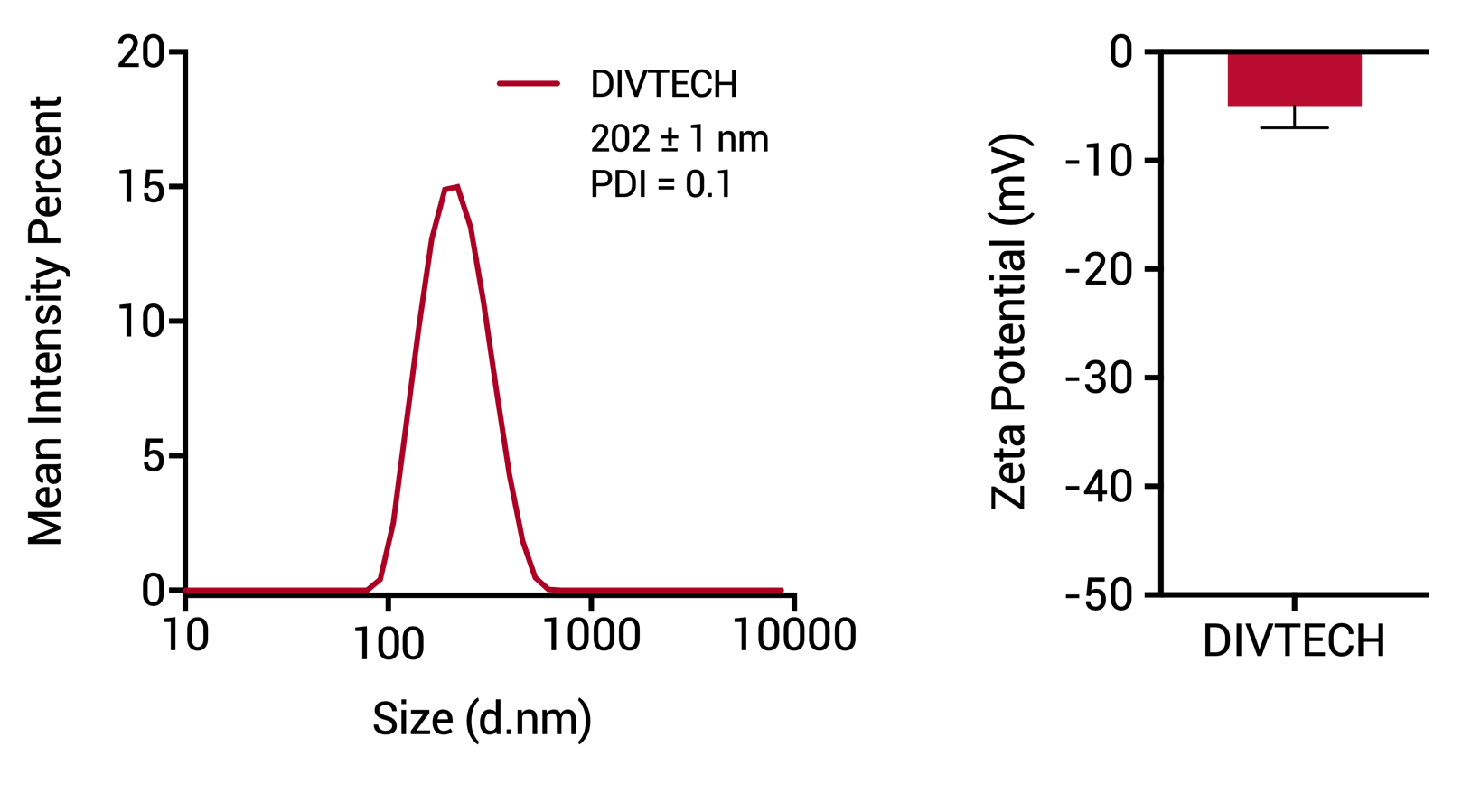
Characterization of size and surface charge expressed as zeta potential, measured by Dynamic Light Scattering (DLS) and ALD.
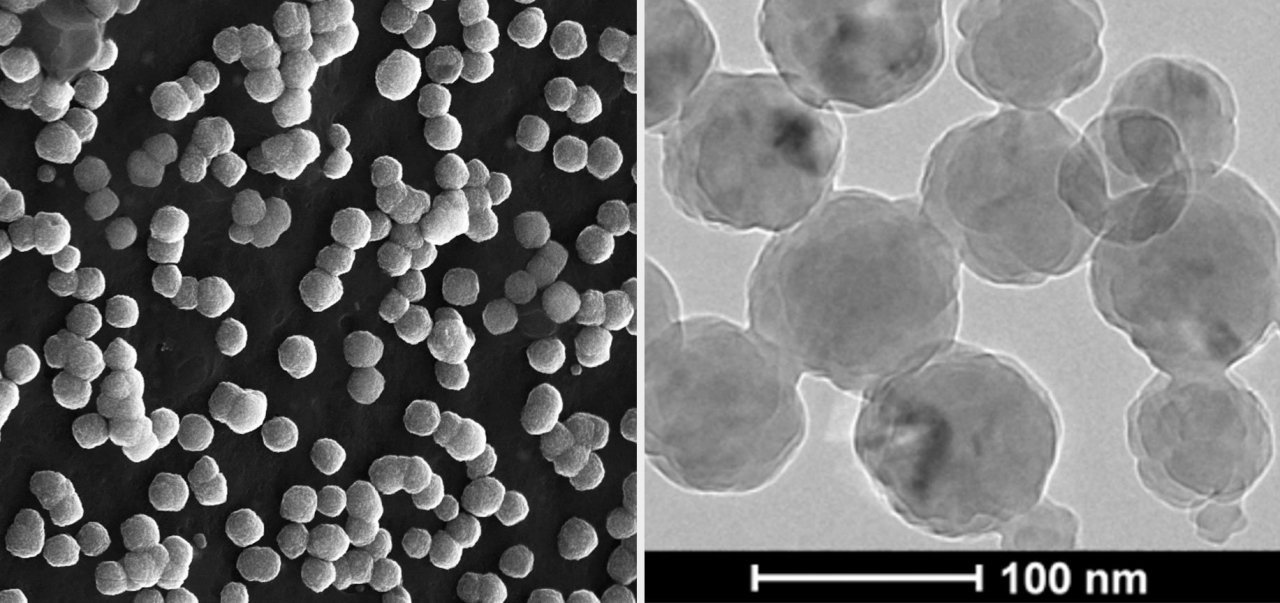 Morphology of DIVERSA’s technology observed by Scanning Transmission Electron Microscopy (STEM) and Cryogenic Transmission Electron Microscopy, Cryo-TEM.
Morphology of DIVERSA’s technology observed by Scanning Transmission Electron Microscopy (STEM) and Cryogenic Transmission Electron Microscopy, Cryo-TEM.
| Cell internalization in fibroblast of DIVTECH labeled with FluoGreen observed under confocal microscopy after 4 hour incubation. | Cell internalization in H1650 spheroids of DIVTECH labeled in red observed under confocal microscopy after 6 hour incubation. |
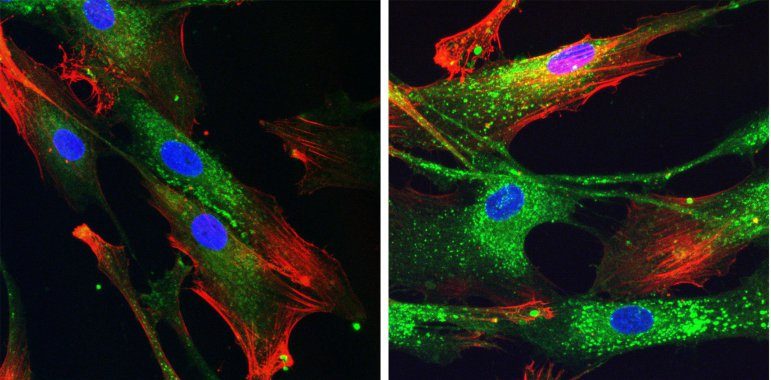
Blue: nuclei, red: fibronectin, green: DIVTECH. |
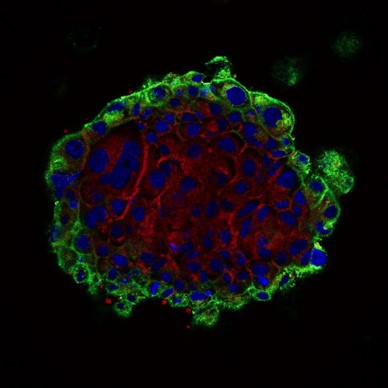
Blue: nuclei, red: DIVTECH-Cy5, green: cell membranes. |
| Human plasma | Simulated tear fluid | Simulated synovial fluid | Simulated gastric fluid | Simulated intestinal fluid (SIF) | Fed state SIF | Fasted state SIF | |
|---|---|---|---|---|---|---|---|
DIVTECH |
24 h* |
24 h* |
24 h* |
6 h |
4 h |
4 h |
24 h* |
*End of the experiment, stable particles.
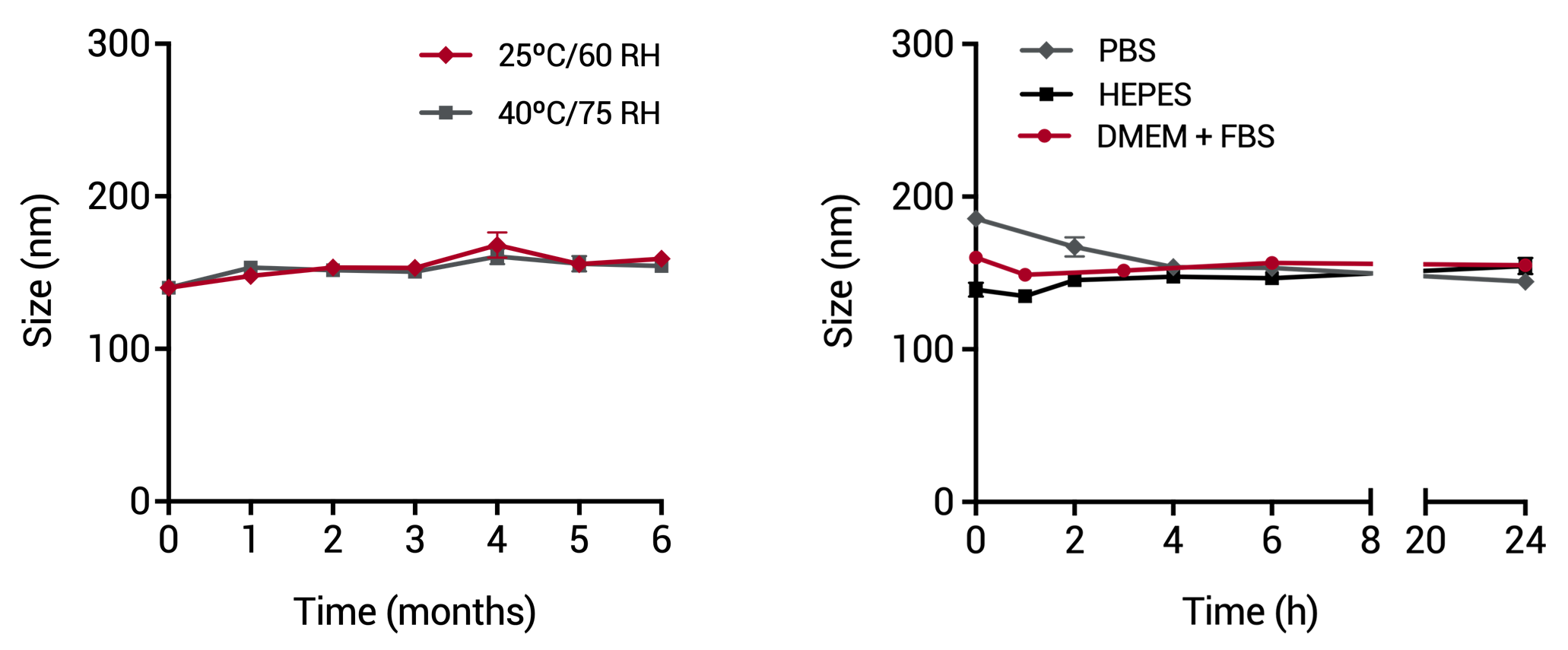
Long-term stability at room temperature (40 ºC, 75% RH) and under storage conditions (25 ºC, 60% RH) up to 6 months, and in different buffers and in culture media supplemented with FBS.
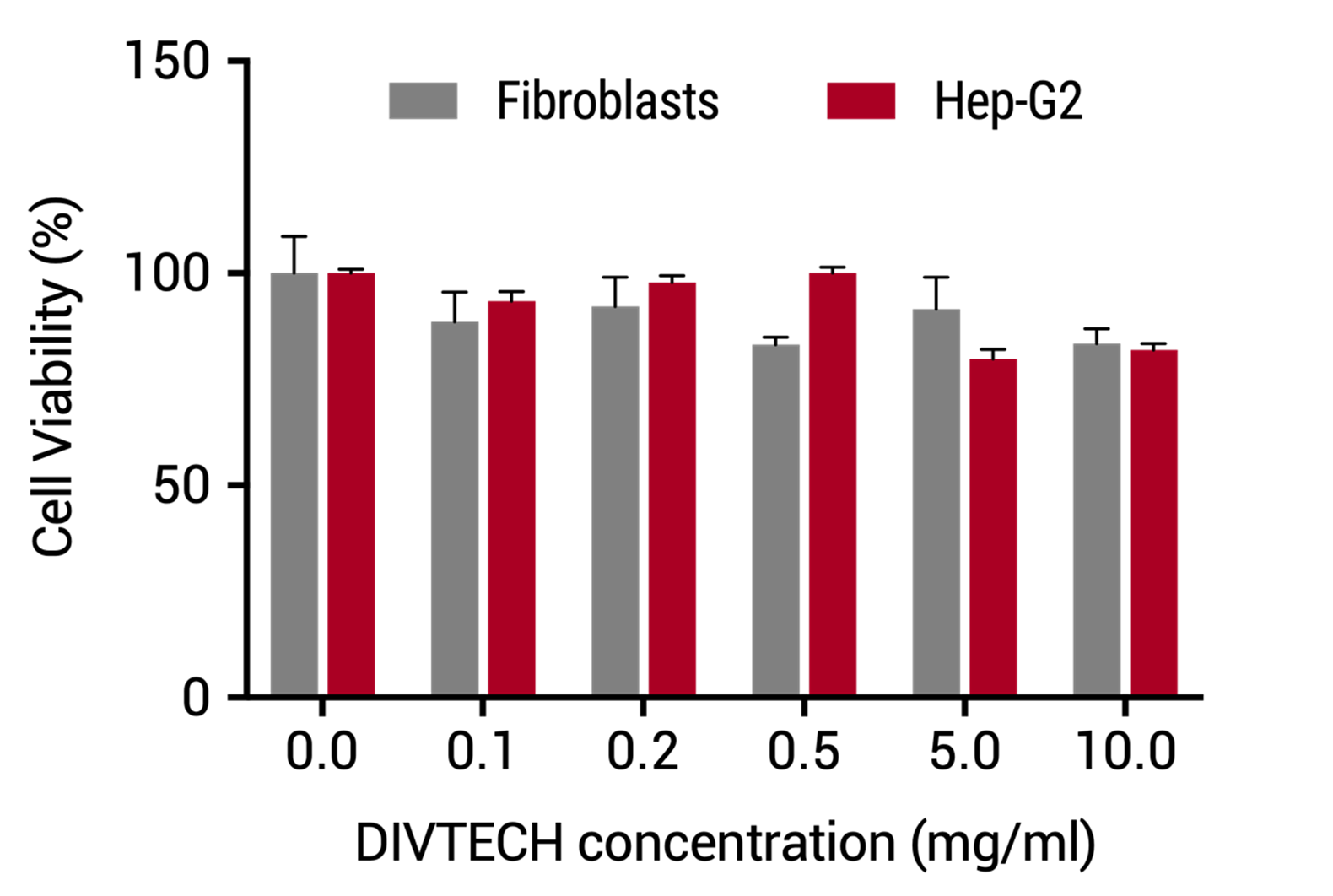
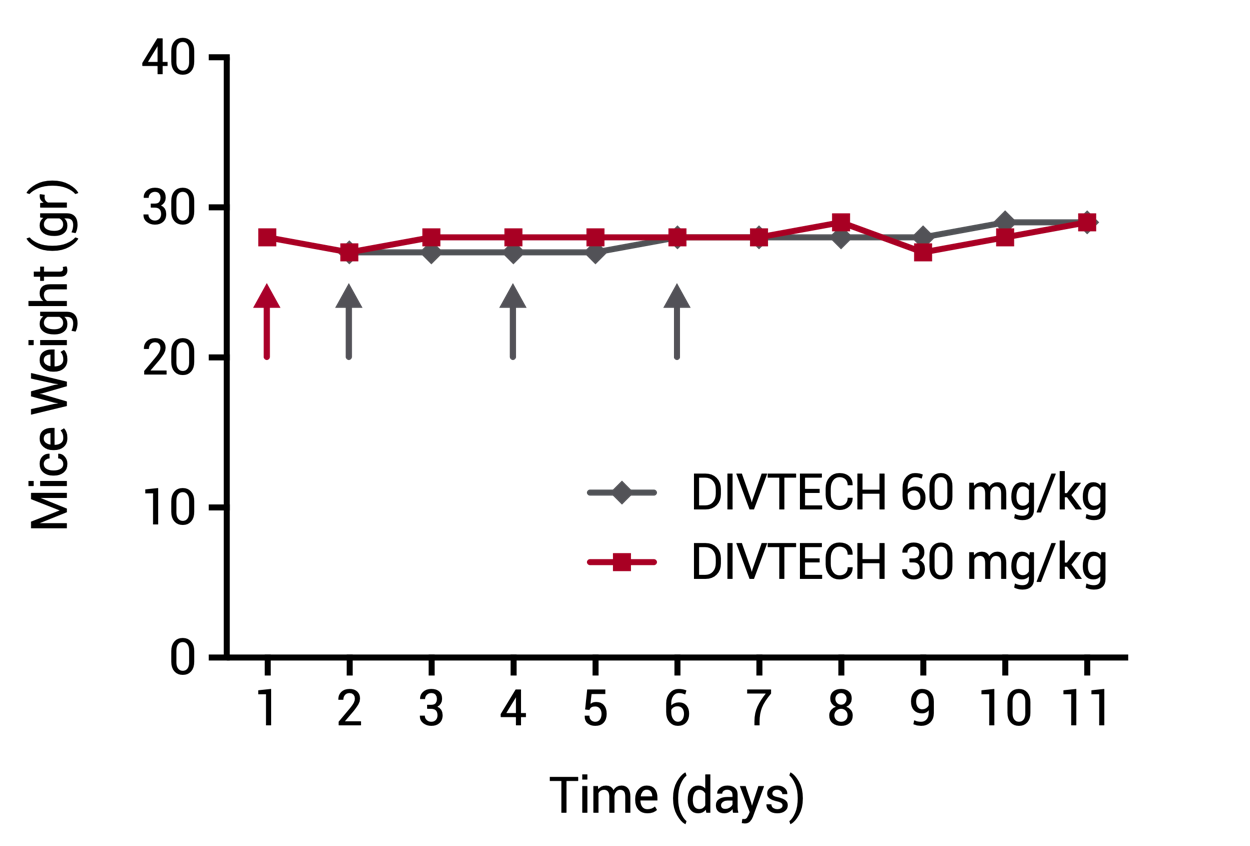
| DIVERSA’s technology showed a great biocompatibility with different cell types and increasing concentrations of the nanosystems measured by Alamar assay. | Evolution of body weight in mice injected with DIVTECH. Red arrow: one dose tail intravenous injection of 30 mg/kg (10 mg/mL DIVTECH concentration). Gray arrows: three consecutive dose tail intravenous injection of 60 mg/kg (20 mg/mL nanosystem concentration) at days 2, 4 and 6 (cumulative dose 180 mg/kg). |
 |
 |
| Molecules | Association efficiency |
|---|---|
Small molecules |
85-99 % |
Peptides |
70-99 % |
Proteins |
70-99 % |
DNA/RNA |
85-99 % |
Publications
Latest work from our team
-
Masoumi F et al (2021). Modulation of Colorectal Tumor Behavior via lncRNA TP53TG1-Lipidic Nanosystem. Pharmaceutics 13(9), 1507. 3390/pharmaceutics13091507
-
Díez-Villares et al. (2021). Biodistribution of 68/67Ga-Radiolabeled Sphingolipid Nanoemulsions by PET and SPECT Imaging. Int J Nanomedicine 16: 5923–5935. 10.2147/IJN.S316767
-
S. Díez-Villares et al. (2021). Manganese Ferrite Nanoparticles Encapsulated into Vitamin E/Sphingomyelin Nanoemulsions as Contrast Agents for High-Sensitive Magnetic Resonance Imaging. Adv Healthc Mater e2101019. 10.1002/adhm.202101019
-
L. Bouzo et al. (2021). Sphingomyelin nanosystems loaded with uroguanylin and etoposide for treating metastatic colorectal cancer. Sci Rep 11: 17213. 10.1038/s41598-021-96578-z
-
L. Bouzo et al. (2020). In Vitro–In Silico Modeling Approach to Rationally Designed Simple and Versatile Drug Delivery Systems. J. Phys. Chem. B 124, 28, 5788–5800. 10.1021/acs.jpcb.0c02731
-
Nagachinta et al. (2020). Sphingomyelin-Based Nanosystems (SNs) for the Development of Anticancer miRNA Therapeutics. Pharmaceutics 12(2), 189. 10.3390/pharmaceutics12020189
