The Food and Drug Administration (FDA) has joined the UK medicines regulator and has approved the first CRISPR-Cas9 gene-editing therapy to treat sickle-cell disease [1]. UK expands approval to β-thalassemia.
The approval of Casgevy, the therapy developed by Vertex and CRISPR Therapeutics, is the first CRISPR therapy approved in the world. It represents a milestone for biotechnology and materializes the promised revolution by gene-editing therapies.
CRISPR-Cas9 awakens fetal hemoglobin from dormancy to treat hemoglobinopathies
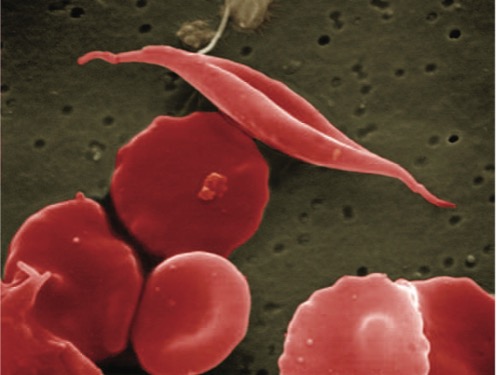
Mutations in the β subunit of hemoglobin, the oxygen-carrying protein in red blood cells, cause β-hemoglobinopathies. In β-thalassemia, the lack of the protein leaves patients with little oxygen-carrying capacity, requiring regular blood transfusions. In sickle cell disease, the mutation causes deformation and clumping of red blood cells, which triggers painful vaso-occlusive inflammatory crises and hemolysis, the bursting of red blood cells, leading to anemia and chronic organ damage.
Image 1. Sickle-shaped red blood cells present in sickle cell disease. Source: National Heart, Lung, and Blood Institute (NHLBI).
The developed treatment is an ex vivo approach. Hematopoietic stem cells are obtained from the patient’s peripheral blood and genetically modified in the laboratory using CRISPR/Cas9, which are introduced into the cells by electroporation. The modification activates the fetal hemoglobin gene to produce proteins that can compensate for the defective β-globin in both β-hemoglobinopathies.
The patients then undergo a bone marrow ablation treatment to remove the dysfunctional blood cells and are injected with the edited stem cells to give rise to functional red blood cells. It is a one-time treatment that has granted complete relief of debilitating episodes of pain for at least one year after treatment in 28 out of 29 participants in the sickle cell trial.
Electroporation is the chosen method to introduce the gene-editing machinery inside the cells. It has been studied for many decades; however, this delivery method can produce irreparable membrane damage and loss of cellular content, and it is not a suitable method for delivering gene therapies for in vivo approaches. Viral vectors and non-viral delivery methods, such as lipid nanoparticles (LNPs), stand as alternatives to grant the future of CRISPR therapies to modify cells in vivo.
What if we go one step further? Can we combine gene editing therapies with nanotechnology?
Nanotechnology can take gene-editing therapies a step further
The direct injection of nucleic acids (plasmid DNA or mRNA encoding Cas9) for in vivo gene editing faces multiple limitations. DNA and RNA are large, negatively charged, and unstable biomolecules that cannot cross lipid bilayers independently. Their short half-life in circulation prevents access to specific target tissues.
Viral vectors as delivery methods remain highly expensive and present poor-tissue selectivity, associated liver toxicity risk, and immunogenic reactions to second doses. This paves the way for non-viral vehicle alternatives, such as lipid nanoparticles.
Lipid nanoparticles allow protection and enhanced delivery of the gene-editing machinery, granting sufficient circulation time to reach target tissues and edit the genome [2]. Not only for CRISPR-based gene therapy but also for mRNA therapies. LNPs mRNA delivery has already demonstrated in COVID-19 vaccines that it is attainable to inoculate repeat doses without compromising patients’ safety. In fact, LNPs are presently being used for CRISPR/Cas9 delivery in two clinical trials to treat hereditary angioedema and transthyretin amyloidosis with cardiomyopathy, both by Intellia Therapeutics (December 2023 data).
Although there is still much room for improvement in the composition of LNPs for repeated dosing, LNPs offer unique functionalities that allow for precise manipulation of the protein corona, granting targeted delivery. LNPs provide a great opportunity to safely deliver mRNA to turn T cells into T regulatory cells ex vivo to treat autoimmune disorders [3].
DIVERSA has focused its R&D efforts on developing lipid nanoparticles suitable for carrying gene editing molecules. Our technology allows for precise modulation by optimizing lipid composition and decoration with proteins and ligands for targeting purposes.
CRISPR components in various formats can be delivered using DIVERSA’s lipid nanosystems, from mRNA encoding Cas9 and gDNA to Cas9/sgRNA combinations.
DIVERSA’s highly adaptable lipid nanoparticles are designed to deliver peptides, proteins, nucleic acids, and small molecules at the intracellular level precisely without compromising function.
We can tailor our technology to your needs by customizing our reagents for gene-editing therapy delivery or by carrying out a co-development agreement.
Benefit from our experience and know-how to deliver your gene-editing therapy. Contact us!